Australian Advantage
Speed, Cost, Quality

Discover the Benefits of Choosing Australia
Australia is a destination of choice for early-phase clinical studies due to its fast and pragmatic regulatory environment, internationally recognised quality systems, and cost competitiveness. These attributes are often termed Speed, Cost, and Quality.
Speed
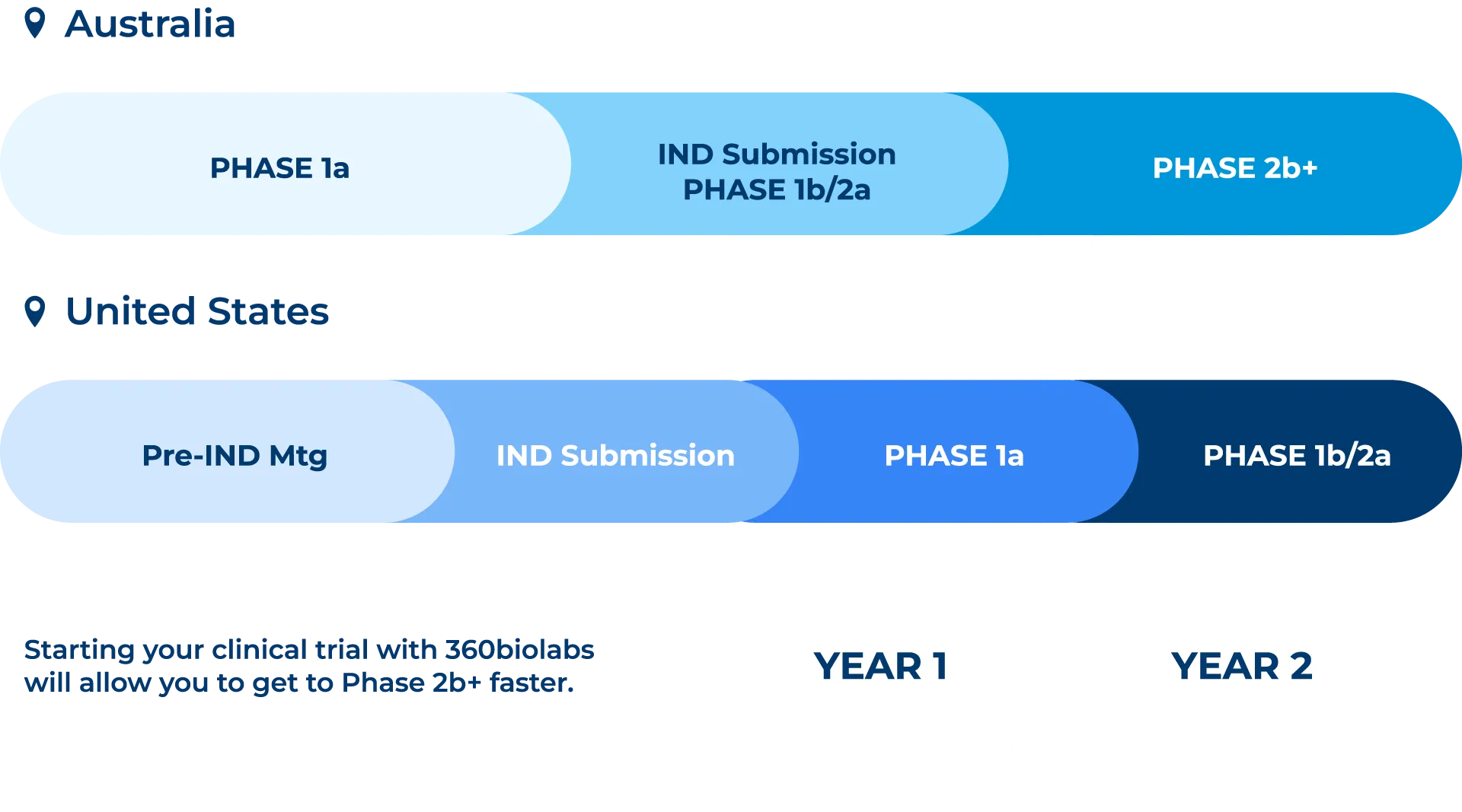
An IND is not required to conduct a clinical study in Australia. Australia offers a fast and pragmatic regulatory environment for early-phase clinical studies with the approval of healthy volunteer studies in ~4-6 weeks.Cost
The Australian government offers an attractive R&D tax incentive of 43.5% to eligible companies.Quality
Australia is an OECD country and a member of the OECD Mutual Acceptance of Data (MAD) system. Data from studies conducted in Australia can be used to support international regulatory applications, including but not limited to the US FDA and European Medicines Evaluation Agency (EMEA).Australian vs. United States Timelines for First In Human Clinical Studies
Australia as a Destination for Clinical Trials
- Trusted Established Market
- Streamlined Regulatory Environment
- Efficient HREC Approval Process
- Robust Data and Intellectual Property Privacy Laws
- World-class Research Institutions and Clinical Trial Facilities
- Diverse Multicultural Participant Population
- Favourable Climate Seasonal Differences to Northern Hemisphere Expedites Vaccine Clinical Trials
- Access To KOL and Experts in Various Medical and Scientific Fields
Australias Leadership
in Healthcare and
Innovation Sectors
- Australia has 5 of the top 50 global medical schools and is one of the leading contributors to life sciences research.
- The favourable exchange rate, which can be as much as 30% more cost-effective, combined with the R&D tax incentive, makes conducting clinical trials in Australia approximately 60% more cost-effective than in the United States.
- Access To KOL, Australia has a pool of key opinion leaders and experts in various medical and scientific fields.
- Australia's ranking as the third-highest recipient of offshore clinical trials in 2021 underscores its strong healthcare infrastructure, skilled workforce, and favourable regulatory environment. These factors make the country an attractive destination for international pharmaceutical and biotechnology companies looking to conduct clinical research.


